

Publications
Janus Cross-links in Supramolecular Networks
Swagata Mondal, Jacob J. Lessard, Chhuttan L. Meena, Gangadhar J. Sanjayan and Brent S. Sumerlin
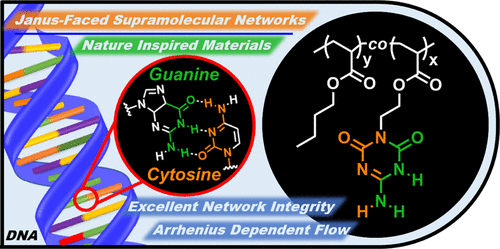
Thermosets composed of cross-linked polymers demonstrate enhanced thermal, solvent, chemical, and dimensional stability as compared to their non-cross-linked counterparts. However, these often-desirable material properties typically come at the expense of reprocessability, recyclability, and healability. One solution to this challenge comes from the construction of polymers that are reversibly cross-linked. We relied on lessons from Nature to present supramolecular polymer networks comprised of cooperative Janus-faced hydrogen bonded cross-links. A triazine-based guanine-cytosine base (GCB) with two complementary faces capable of self-assembly through three hydrogen bonding sites was incorporated into poly(butyl acrylate) to create a reprocessable and recyclable network. Rheological experiments and dynamic mechanical analysis (DMA) were employed to investigate the flow behavior of copolymers with randomly distributed GCB units of varying incorporation. Our studies revealed that the cooperativity of multiple hydrogen bonding faces yields excellent network integrity evidenced by a rubbery plateau that spanned the widest temperature range yet reported for any supramolecular network. To verify that each Janus-faced motif engages in multiple cross-links, we studied the effects of local concentration of the incorporated GCB units within the polymer chain. Mechanical strength improved by colocalizing the GCB within a block copolymer morphology. This enhanced performance revealed that the number of effective cross-links in the network increased with the local concentration of hydrogen bonding units. Overall, this study demonstrates that cooperative noncovalent interactions introduced through Janus-faced hydrogen bonding moieties confers excellent network stability and predictable viscoelastic flow behavior in supramolecular networks.
Tuning Supramolecular Polymer Assembly through Stereoelectronic Interactions
Will R. Henderson, Guancen Liu, Khalil A. Abboud and Ronald K. Castellano

The supramolecular polymerization of 2,11-dithia[3.3]paracyclophanes through self-complementary intermolecular and transannular amide hydrogen bonding is presented. An n → π* interaction between the amide hydrogen bonding units and the central bridging atom results from the single-point exchange of a carbon atom for a sulfur atom. This orbital donor–acceptor interaction can be strengthened by oxidizing the sulfide to a sulfone which acts to shorten the donor···acceptor distance and increase orbital overlap. Experimental signatures of the increased n → π* interaction include larger isodesmic polymerization elongation constants in solution, changes in characteristic bond stretching frequencies, and geometric/structural changes evaluated by X-ray crystallography. The experimental data are supported by extensive computational investigations of both assembling and nonassembling 2,11-dithia[3.3]paracyclophanes as well as a rationally designed model system to confirm the role of stereoelectronic effects on supramolecular polymer assembly.
Journal of the American Chemical Society, 2021, 143, 12688-12698
Adsorption space for microporous polymers with diverse adsorbate species
Dylan M. Anstine, Dai Tang, David S. Sholl & Coray M. Colina

The enormous number of combinations of adsorbing molecules and porous materials that exist is known as adsorption space. The adsorption space for microporous polymers has not yet been systematically explored, especially when compared with efforts for crystalline adsorbents. We report molecular simulation data for the adsorptive and structural properties of polymers of intrinsic microporosity with a diverse set of adsorbate species with 345 distinct adsorption isotherms and over 240,000 fresh and swollen structures. These structures and isotherms were obtained using a sorption-relaxation technique that accounts for the critical role of flexibility of the polymeric adsorbents. This enables us to introduce a set of correlations that can estimate adsorbent swelling and fractional free volume dilation as a function of adsorbate uptake based on readily characterized properties. The separation selectivity of the 276 distinct binary molecular pairs in our data is reported and high-performing adsorbent systems are identified.
npj Computational Materials, 2021, 7, 53
Cyclic polyacetylene
Zhihui Miao, Stella A. Gonsales, Christian Ehm, Frederic Mentink-Vigier, Clifford R. Bowers, Brent S. Sumerlin and Adam S. Veige

Here we demonstrate the synthesis of cyclic polyacetylene (c-PA), or [∞]annulene, via homogeneous tungsten-catalysed polymerization of acetylene. Unique to the cyclic structure and evidence for its topology, the c-PA contains >99% trans double bonds, even when synthesized at −94 °C. High activity with low catalyst loadings allows for the synthesis of temporarily soluble c-PA, thus opening the opportunity to derivatize the polymer in solution. Absolute evidence for the cyclic topology comes from atomic force microscopy images of bottlebrush derivatives generated from soluble c-PA. Now available in its cyclic form, initial characterization studies are presented to elucidate the topological differences compared with traditionally synthesized linear polyacetylene. One advantage to the synthesis of c-PA is the direct synthesis of the trans–transoid isomer. Low defect concentrations, low soliton concentration, and relatively high conjugation lengths are characteristics of c-PA. Efficient catalysis permits the rapid synthesis of lustrous flexible thin films of c-PA, and when doped with I , they are highly conductive (398 (±76) Ω cm ).
2
-1
-1
ADMET polymers: synthesis, structure elucidation, and function
Julia Pribyl, Kenneth B. Wagener and Giovanni Rojas

Acyclic Diene Metathesis polycondensation produces materials with well-defined primary structures, perfection that is the consequence of symmetry imparted by monomer design. Diverse functionalities can be incorporated either in the polymer backbone or as pendant groups; chemical compatibility of the functional group with the catalyst is a requirement. Structural perfection led to structure/property investigations that would be difficult in imprecise systems. This comprehensive review summarizes the synthetic strategies for production of ADMET polymers. Further presented are the effects on secondary structure of controlling branch identity, in-chain functional groups, and frequency along the polymer chain. Results of spectroscopic, thermal and imaging techniques have been key for understanding the relationships between structure and properties.
Materials Chemistry Frontiers, 2021,5, 14-43
Thermodynamics of silica depolymerization with alcohols
Jordan L. Torgunrud, Alejandro J. Faria and Stephen A. Miller

Experimental and computational results describe the outlook for silica (polymeric SiO ) depolymerization with alcohols. The resonance energy of the Si-O-Si segment, calculated as 8.0 kcal/mol, or 16.0 kcal/mol of SiO , explains the thermodynamic stability of silica. Acid-catalyzed alkylorthosilicate metathesis reactions between Si(OMe) and diols indicate silicon’s thermodynamic preference for diols because of favorable entropic chelation. Conversion to Si(OCH CH O) with ethylene glycol is 74.6% and conversion to Si(OCH CH CH O) with 1,3-propanediol is 66.2%. The enthalpic stability of Si(OR) species correlates to two main factors: (1) the O-Si-O bond angle, with a compression force constant of k = 0.0315 (kcal/mol)(°) and expansion force constant of k = 0.0167 (kcal/mol)(°) ; and (2) the C-O-Si-O dihedral angle, dictated by stabilizing O n→ Si-O σ* anomeric interactions of 3.5 kcal/mol in the model compound Si(OMe) . Computationally, silica depolymerization with methanol is never exergonic, but depolymerization with 1,3-propanediol, forming Si(OCH CH CH O) and two equivalents of water, is exergonic above 99°C in water.
2
2
4
2
2
2
2
2
2
2
4
Θ-comp
Θ-exp
-2
-2
4
2
2
2
2
Polyhedron, 2020, 187, 114562